Certivo for the Medical Devices Industry
Certivo for the Medical Devices Industry
Automate, Streamline and Simplify Compliance Management
Simplify Compliance Without Compromising on Safety or Speed
Simplify Compliance Without Compromising on Safety or Speed
Automate, Streamline and Simplify Compliance Management
Medical Device Compliance manufacturers face some of the world’s strictest regulatory requirements. Compliance is non-negotiable — but it’s also a significant bottleneck.
Manual document collection, changing global regulations, and demanding customers create constant delays and risk.
Certivo helps medical device teams streamline compliance, eliminate documentation gaps, and launch confidently — faster.
Medical Device Compliance manufacturers face some of the world’s strictest regulatory requirements. Compliance is non-negotiable — but it’s also a significant bottleneck.
Manual document collection, changing global regulations, and demanding customers create constant delays and risk.
Certivo helps medical device teams streamline compliance, eliminate documentation gaps, and launch confidently — faster.
Medical Device Compliance manufacturers face some of the world’s strictest regulatory requirements. Compliance is non-negotiable — but it’s also a significant bottleneck.
Manual document collection, changing global regulations, and demanding customers create constant delays and risk.
Certivo helps medical device teams streamline compliance, eliminate documentation gaps, and launch confidently — faster.
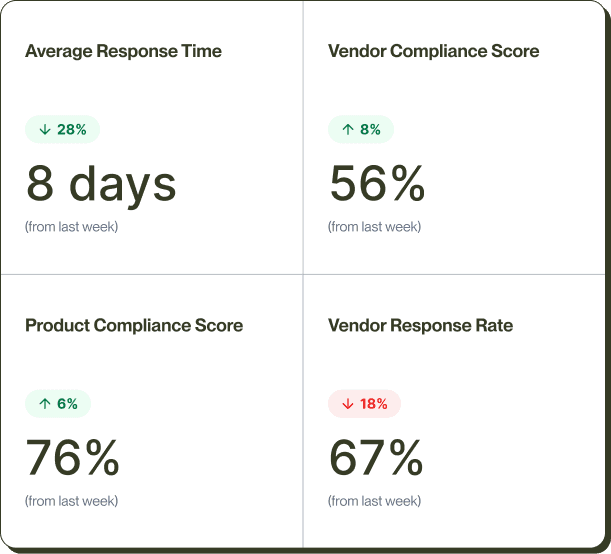

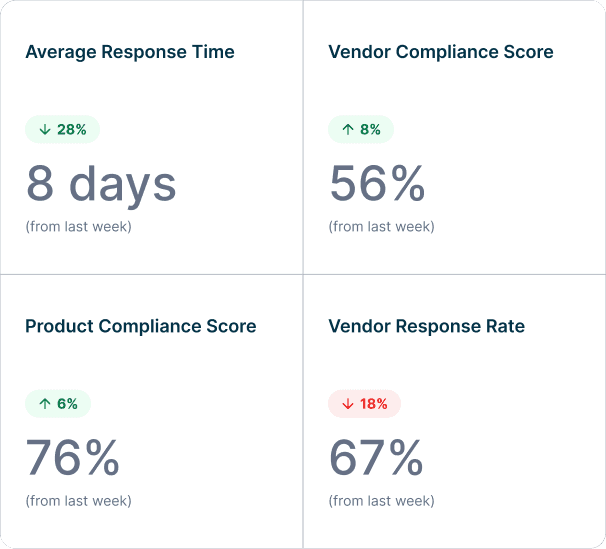
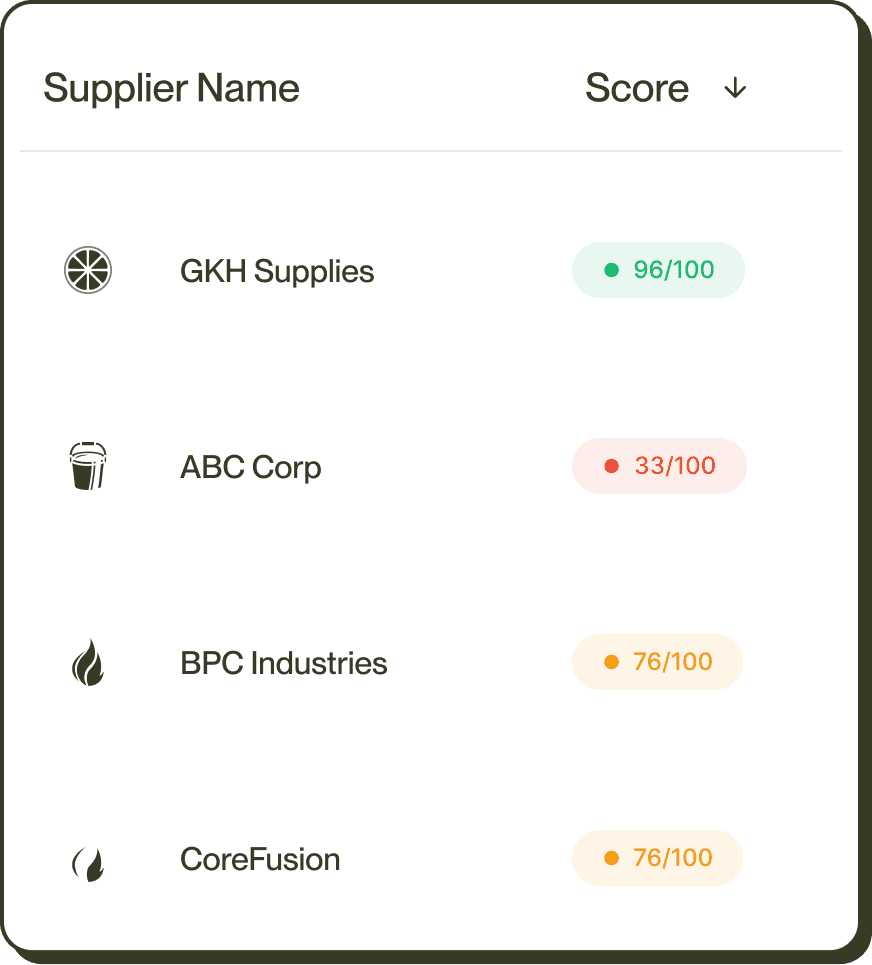


CORA Update
CORA verifying Resistor #2828 RoHS certificate from GHK Supplies
CORA Update
CORA verifying Resistor #2828 RoHS certificate from GHK Supplies
CORA Update
CORA verifying Resistor #2828 RoHS certificate from GHK Supplies
Constantly Evolving Regulatory RequirementsYou’re navigating multiple frameworks:
Constantly Evolving Regulatory RequirementsYou’re navigating multiple frameworks:
IATF 16949 and ISO 26262 (for quality/safety alignment)
Conflict Minerals
RoHS, REACH, TSCA, Prop 65
IMDS material reporting
Supplier-specific and OEM-specific declaration formats
ELV Directive (EU)
Meeting Scheduler
Certivo keeps track of all of them — automatically monitoring changes, highlighting affected products, and alerting your team with clear next steps.
Constantly Evolving Regulatory RequirementsYou’re navigating multiple frameworks:
Constantly Evolving Regulatory RequirementsYou’re navigating multiple frameworks:
IATF 16949 and ISO 26262 (for quality/safety alignment)
Conflict Minerals
RoHS, REACH, TSCA, Prop 65
IMDS material reporting
Supplier-specific and OEM-specific declaration formats
ELV Directive (EU)
Meeting Scheduler
Certivo keeps track of all of them — automatically monitoring changes, highlighting affected products, and alerting your team with clear next steps.
Constantly Evolving Regulatory RequirementsYou’re navigating multiple frameworks:
Constantly Evolving Regulatory RequirementsYou’re navigating multiple frameworks:
IATF 16949 and ISO 26262 (for quality/safety alignment)
Conflict Minerals
RoHS, REACH, TSCA, Prop 65
IMDS material reporting
Supplier-specific and OEM-specific declaration formats
ELV Directive (EU)
Meeting Scheduler
Certivo keeps track of all of them — automatically monitoring changes, highlighting affected products, and alerting your team with clear next steps.
Burden of Documentation
Certivo Ensure EU MDR Compliance System so that medical devices meet the regulatory standards in the European Market. From BOMs and SDSs to Declarations of Conformity and test reports — missing or incorrect documentation leads to failed audits, blocked shipments, or loss of trust.
Step 1
Supplier Certificates
We assess your needs and identify AI solutions to streamline workflows and improve efficiency.
Step 2
Full Material Declarations (FMDs)
Our team builds intelligent automation systems tailored to your business processes.
Step 3
Safety Data Sheets (SDSs)
We smoothly integrate AI solutions into your existing infrastructure with minimal disruption.
Step 4
Country or customer-specific compliance forms and ISO 13485 documentation
We refine performance, analyze insights, and enhance automation for long-term growth.
Case Studies
Case Studies
Case Studies
See How Smart AI Automation Transforms Businesses
See how AI automation streamlines operations, boosts and drives growth.

Multi-Tier Supplier Complexity
Your compliance depends on documentation from hundreds of suppliers — often Tier 2 or Tier 3 — who aren’t always responsive with FDA 21 CFR part 820
Certivo drives:
Basic workflow automation
90% supplier response rates
Automated outreach and follow-up
Language translation and communication management
Centralized dashboards to track responsiveness by supplier and product
Basic workflow automation

What Certivo Unlocks for Medical Device Teams
Impact :
Basic workflow automation
Faster product launches and market access
Streamlined audits and inspections
Full traceability from material to finished device
Proactive regulatory alerts and updates
Time savings for RA/QA teams by 80–90%
Confidence in documentation during FDA, Notified Body, or internal reviews under FDA 21 CFR part 820

How Certivo Works With You
CORA, our intelligent compliance agent, helps you:
Collect supplier certifications with intelligent automation
Monitor frameworks like EU MDR Compliance, ISO 13485, RoHS, and REACH
Parse and organize documents into audit-ready formats
Flag at-risk parts and expired certs in real time
Centralized dashboards to track responsiveness by supplier and product

Built for These Use Cases
Implantables & disposables
Diagnostic and laboratory equipment
Wearable health tech
Imaging systems
Hospital-grade electronics
Devices integrating electronics and sensors ensure Compliance with software for medical devices.

Multi-Tier Supplier Complexity
Your compliance depends on documentation from hundreds of suppliers — often Tier 2 or Tier 3 — who aren’t always responsive with FDA 21 CFR part 820
Certivo drives:
Basic workflow automation
90% supplier response rates
Automated outreach and follow-up
Language translation and communication management
Centralized dashboards to track responsiveness by supplier and product
Basic workflow automation

What Certivo Unlocks for Medical Device Teams
Impact :
Basic workflow automation
Faster product launches and market access
Streamlined audits and inspections
Full traceability from material to finished device
Proactive regulatory alerts and updates
Time savings for RA/QA teams by 80–90%
Confidence in documentation during FDA, Notified Body, or internal reviews under FDA 21 CFR part 820

How Certivo Works With You
CORA, our intelligent compliance agent, helps you:
Collect supplier certifications with intelligent automation
Monitor frameworks like EU MDR Compliance, ISO 13485, RoHS, and REACH
Parse and organize documents into audit-ready formats
Flag at-risk parts and expired certs in real time
Centralized dashboards to track responsiveness by supplier and product

Built for These Use Cases
Implantables & disposables
Diagnostic and laboratory equipment
Wearable health tech
Imaging systems
Hospital-grade electronics
Devices integrating electronics and sensors ensure Compliance with software for medical devices.

Multi-Tier Supplier Complexity
Your compliance depends on documentation from hundreds of suppliers — often Tier 2 or Tier 3 — who aren’t always responsive with FDA 21 CFR part 820
Certivo drives:
Basic workflow automation
90% supplier response rates
Automated outreach and follow-up
Language translation and communication management
Centralized dashboards to track responsiveness by supplier and product
Basic workflow automation

What Certivo Unlocks for Medical Device Teams
Impact :
Basic workflow automation
Faster product launches and market access
Streamlined audits and inspections
Full traceability from material to finished device
Proactive regulatory alerts and updates
Time savings for RA/QA teams by 80–90%
Confidence in documentation during FDA, Notified Body, or internal reviews under FDA 21 CFR part 820

How Certivo Works With You
CORA, our intelligent compliance agent, helps you:
Collect supplier certifications with intelligent automation
Monitor frameworks like EU MDR Compliance, ISO 13485, RoHS, and REACH
Parse and organize documents into audit-ready formats
Flag at-risk parts and expired certs in real time
Centralized dashboards to track responsiveness by supplier and product

Built for These Use Cases
Implantables & disposables
Diagnostic and laboratory equipment
Wearable health tech
Imaging systems
Hospital-grade electronics
Devices integrating electronics and sensors ensure Compliance with software for medical devices.

Multi-Tier Supplier Complexity
Your compliance depends on documentation from hundreds of suppliers — often Tier 2 or Tier 3 — who aren’t always responsive with FDA 21 CFR part 820
Certivo drives:
Basic workflow automation
90% supplier response rates
Automated outreach and follow-up
Language translation and communication management
Centralized dashboards to track responsiveness by supplier and product
Basic workflow automation

What Certivo Unlocks for Medical Device Teams
Impact :
Basic workflow automation
Faster product launches and market access
Streamlined audits and inspections
Full traceability from material to finished device
Proactive regulatory alerts and updates
Time savings for RA/QA teams by 80–90%
Confidence in documentation during FDA, Notified Body, or internal reviews under FDA 21 CFR part 820

How Certivo Works With You
CORA, our intelligent compliance agent, helps you:
Collect supplier certifications with intelligent automation
Monitor frameworks like EU MDR Compliance, ISO 13485, RoHS, and REACH
Parse and organize documents into audit-ready formats
Flag at-risk parts and expired certs in real time
Centralized dashboards to track responsiveness by supplier and product

Built for These Use Cases
Implantables & disposables
Diagnostic and laboratory equipment
Wearable health tech
Imaging systems
Hospital-grade electronics
Devices integrating electronics and sensors ensure Compliance with software for medical devices.
DRAG TO EXPLORE
DRAG TO EXPLORE

"AI-driven forecasting cut inventory waste by 40% for TrailForge"
TrailForge, a suitcase brand, faced stock issues and inefficiencies. Our AI forecasting optimized inventory and production cycles, helping them save costs and deliver faster.
Impact :
40% Less Inventory Waste
35% Faster Production
20% More Accurate Forecasting
25% Faster Fulfillment

"AI-driven forecasting cut inventory waste by 40% for TrailForge"
TrailForge, a suitcase brand, faced stock issues and inefficiencies. Our AI forecasting optimized inventory and production cycles, helping them save costs and deliver faster.
Impact :
40% Less Inventory Waste
35% Faster Production
20% More Accurate Forecasting
25% Faster Fulfillment

"AI-powered workflows reduced error rate by 80% in daily operations"
MedixChain, a healthcare logistics company, was dealing with frequent data errors and delays. We introduced AI validation and live tracking to improve accuracy and speed across their supply chain.
Impact :
80% Error reduction
90% Accuracy in Data Logs
30% Faster Delivery
60+ Hours Saved

"AI-powered workflows reduced error rate by 80% in daily operations"
MedixChain, a healthcare logistics company, was dealing with frequent data errors and delays. We introduced AI validation and live tracking to improve accuracy and speed across their supply chain.
Impact :
80% Error reduction
90% Accuracy in Data Logs
30% Faster Delivery
60+ Hours Saved

"AI integration helped ScaleByte close 3x more deals in less time"
ScaleByte’s sales team struggled with follow-up delays. Our AI sales assistant automated outreach, lead scoring, and CRM updates—resulting in faster responses and more closed deals.
Impact :
3x More Deals
40% Faster Responses
95% Lead Accuracy
CRM Fully Synced

"AI integration helped ScaleByte close 3x more deals in less time"
ScaleByte’s sales team struggled with follow-up delays. Our AI sales assistant automated outreach, lead scoring, and CRM updates—resulting in faster responses and more closed deals.
Impact :
3x More Deals
40% Faster Responses
95% Lead Accuracy
CRM Fully Synced

"Automating 50% of operations saved 20% in costs in 2 months"
FinSolve, a financial services firm, was overloaded with repetitive tasks. By automating workflows and integrating data systems, they streamlined operations and significantly reduced overhead.
Impact :
50% Operations Automated
20% Cost Reduction
70+ Hours Saved/Month
2x Faster Client Onboarding

"Automating 50% of operations saved 20% in costs in 2 months"
FinSolve, a financial services firm, was overloaded with repetitive tasks. By automating workflows and integrating data systems, they streamlined operations and significantly reduced overhead.
Impact :
50% Operations Automated
20% Cost Reduction
70+ Hours Saved/Month
2x Faster Client Onboarding